|
Controlled Ovarian Hyperstimulation: Pergonal #5
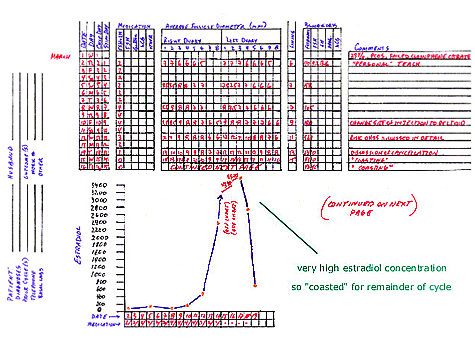
This (successful) cycle of controlled ovarian hyperstimulation (COH) with intrauterine insemination (IUI) illustrates the
progress that may occur during some COH cycles characterized by very high estradiol concentrations. Comments about this particular
cycle might include:
1. The pretreatment diagnosis of polycystic ovarian syndrome (PCOS) included the classic elevation in LH to FSH ratio of greater than 3 to 1. The LH concentration was 13.6 IU/L with a FSH concentration of 4.2 IU/L. Additional clinical signs of PCOS include hirsutism (excessive hair growth in “male pattern” areas of the body), acne and oily skin, amenorrhea (absence of menstrual flow, with vaginal bleeding possibly every 1-2 years without medications) due to anovulation (absence of ovulation of mature eggs), and android obesity (excess weight in male pattern areas like the waist rather than the hips).
2. This patient failed to ovulate to maximal doses of clomiphene citrate. Insulin resistance is often increased in PCOS women, and if identified then use of insulin sensitizing medication may be considered (these medications have been associated with an improved ability to induce ovulation with clomiphene citrate). This woman had normal insulin tolerance testing. Therefore, the decision was made to proceed with ovulation induction with menotropins.
3. I started the patient on a standard dose of menotropins. In my experience, achieving a stimulatory response with an adequate amount of menotropin and then cutting back on the medication has the advantage of “getting things going” and once follicular development results in a few 12-13 mm follicles these will often continue to grow (since they have developed a much greater local blood flow to themselves and a greater number of FSH receptors) even if the medication dose is severely decreased. By decreasing the FSH dosage, the smaller follicles that are less capable of competing for FSH often shrink (and may become less active). The risk of ovarian hyperstimulation syndrome (OHSS) and higher order multiple pregnancies is considerable during these PCOS menotropin cycles. Alternatively, there is a significant group of infertility specialists who propose that starting at very low menotropin doses for extended periods of time (sometimes weeks) will more likely recruit less follicles to mature in these difficult patients. I have not really been trained in this manner and have no experience with these protocols. There is also a significant group of infertility specialists who believe that stimulating the ovaries in these patients is safest by using GnRH itself. This IV medication is often administered using an indwelling intravenous catheter attached to a pump (that is about the size of a transistor (walkman) radio that must be worn continuously for several weeks). The risk of OHSS and higher order multiple pregnancies is almost eliminated using this medication. GnRH pumps are not easily available (in the USA), the IV catheters and the pump are difficult to maintain, and patients often do not like carrying the pump everywhere for several weeks at a time. I don’t have much experience with this treatment option.
4. After three days of stimulation, the estradiol concentration rose to 158 pg/mL, which generally would reflect a good increase.
PCOS women often have an elevated basal estradiol concentration, and indeed this woman had a basal level of 110 pg/mL. The follicular
growth was less than usually seen, but there did seem to be some development. Therefore, the dose was not changed and 3 more days of
medication were suggested.
5. After 6 days of stimulation, the estradiol concentration dropped to 105 pg/mL. The follicular growth was markedly decreased
compared to expected. Therefore, the decision was to increase the menotropin dose to 4 ampules per day for 2 days. More frequent
monitoring is required for PCOS women when there is a change in medication dose since their response to medication can be brittle
(unpredictable). In other words, there might be too much development on 3 ampules a day and no development at all on 2 ampules a day.
6. After 8 days of medication, there was some follicular development and the estradiol increased to 180 pg/mL. Two more days of 4
ampules per day were suggested.
7. After the 10th day of stimulation, the follicules were developing nicely, the lead follicles were about 12mm, and the estradiol
jumped up to 561 pg/mL. The decision was to start cutting back on the medication dose, so 3 ampules were given that day and 2 ampules
were given the following day.
8. After the 12th day of stimulation, there was continued growth of follicles and the estradiol rose dramatically to 2390 pg/mL.
Since the lead follicles were only 13mm diameter it was thought that at least some menotropin would be required so 1 ampule was given
and the patient was monitored the next day.
9. After the 13th day of stimulation medication, the lead follicular diameters grew to 14mm, and the estradiol concentration nearly
doubled to 4310 pg/mL. The risk of OHSS is associated with elevated estradiol concentrations and there appears to be a correlation
between the severity of OHSS and the degree (amount) of estradiol elevation. During COH/IUI cycles, milder forms of OHSS may develop
when hCG is administered to trigger ovulation and the estradiol concentration (on the day of hCG) is in excess of 2,000 pg/mL. More
severe forms of OHSS are more common when ovulation is triggered and the estradiol concentration is greater than 3000 pg/mL. In the
event of an estradiol concentration greater than 3,000 pg/mL during COH/IUI, I either cancel the cycle entirely (the hCG is not given)
or I “coast” the cycle. Coasting a COH/IUI cycle involves withholding further menotropins and allowing remaining follicles to grow
until the estradiol concentration begins to drop and is in a “safe” zone for hCG (trigger of ovulation).
10.After the 14th day from the onset of stimulation, the estradiol concentration continued to rise to 4646 pg/mL. Coasting was
continued.
11.After the 15th day from the onset of stimulation, the estradiol concentration began to fall (3600 pg/mL) and the ultrasound
revealed that the lead follicles were 15.5mm in average diameter. Coasting was continued.
12.After the 16th day, the estradiol concentration was 2760 pg/mL and the lead follicles were 17mm. In this situation, the
estradiol concentration is nearly 3000 pg/mL so coasting was continued. A “safe” level of estradiol concentration in these
circumstances is not clear. Basically, OHSS is caused by an agent that is unknown that either “is” or “is not” produced during controlled ovarian hyperstimulation cycles, and this event produces the cascade of events that result in OHSS. The absolute concentration of estradiol is thought to correlate with OHSS primarily as a marker of an excessive ovarian response and also an abundance of small to midsize follicles. I generally feel comfortable administering hCG to trigger ovulation when the estradiol concentration “takes a deep drop” during coasting, to signify a marked reduction in active follicular events.
13.After the 17th day, the estradiol concentration was 676 pg/mL and the lead follicles were 18.5mm in average diameter. 5,000 IU of hCG was given since a drop to less than 1500 pg/mL in this case was considered a deep drop.
14.The hCG titers were positive at the time of “missed menses” (163 IU/L) and rose appropriately (a little more than doubled in 2 days). At 4 weeks after ovulation (6 weeks gestation) viable triplets were identified in the uterus. I discussed the availability of selective reduction to twin with the couple in detail.
Additional Case Studies: COH Cycle 1 | COH Cycle 2
| COH Cycle 3
| COH Cycle 4 | COH Cycle 5
|

|